Abstract
Purpose
In ventilator-associated pneumonia (VAP), early appropriate antimicrobial therapy may be hampered by involvement of multidrug-resistant (MDR) pathogens.
Methods
A systematic review and diagnostic test accuracy meta-analysis were performed to analyse whether lower respiratory tract surveillance cultures accurately predict the causative pathogens of subsequent VAP in adult patients. Selection and assessment of eligibility were performed by three investigators by mutual consideration. Of the 525 studies retrieved, 14 were eligible for inclusion (all in English; published since 1994), accounting for 791 VAP episodes. The following data were collected: study and population characteristics; in- and exclusion criteria; diagnostic criteria for VAP; microbiological workup of surveillance and diagnostic VAP cultures. Sub-analyses were conducted for VAP caused by Staphylococcus aureus, Pseudomonas spp., and Acinetobacter spp., MDR microorganisms, frequency of sampling, and consideration of all versus the most recent surveillance cultures.
Results
The meta-analysis showed a high accuracy of surveillance cultures, with pooled sensitivities up to 0.75 and specificities up to 0.92 in culture-positive VAP. The area under the curve (AUC) of the hierarchical summary receiver-operating characteristic curve demonstrates moderate accuracy (AUC: 0.90) in predicting multidrug resistance. A sampling frequency of >2/week (sensitivity 0.79; specificity 0.96) and consideration of only the most recent surveillance culture (sensitivity 0.78; specificity 0.96) are associated with a higher accuracy of prediction.
Conclusions
This study provides evidence for the benefit of surveillance cultures in predicting MDR bacterial pathogens in VAP. However, clinical and statistical heterogeneity, limited samples sizes, and bias remain important limitations of this meta-analysis.
Similar content being viewed by others
Introduction
Despite increased efforts in infection prevention, ventilator-associated pneumonia (VAP) remains an important complication in intensive care unit (ICU) patients. A systematic review revealed that VAP develops in 10–20 % of patients receiving mechanical ventilation for more than 48 h [1]. VAP contributes to a substantial economic burden through an added length of ICU stay of about 5–7 days and an attributable cost ranging from $10,000 to $13,650 [1]. Furthermore, patients with VAP appear to be twice as likely to die in the ICU compared with matched control subjects without this complication [2]. However, the study of Timsit et al. [3], highlighting the important heterogeneity among VAP studies, estimated the ‘attributable’ mortality to be about 6 %. Pivotal to optimising the odds of survival is prompt initiation of empiric appropriate therapy [4, 5]. Inappropriate empiric therapy during the critical time frame of the first 24–48 h results in an increased risk of death [6, 7]. The foremost important risk factor for inappropriate empiric antimicrobial therapy is multidrug resistance (MDR) [8]. Prior antimicrobial exposure and prolonged ICU stay are the principal risk factors for MDR [9]. Risk and fear of inappropriate therapy may encourage the use of ‘last-line’ or broad-spectrum antimicrobials or combinations up front, but on the other hand a more restrictive policy of antimicrobial stewardship is advocated [10]. To keep the balance between maximising the rate of appropriate empiric therapy and minimising antimicrobial selection pressure, last-line or broad-spectrum antimicrobials such as carbapenems are recommended in patients with risk factors for infection with MDR pathogens [9, 11]. However, in contrast with the 1990s when the problem of MDR was typically restricted to the ICU, resistance has spread to general wards and spilled over into the community as well. As a result, the predictive value of the risk factors mentioned has become less strong. A recent cohort study in 24 ICUs demonstrated that in ICU patients without classic risk factors, 40 % of nosocomial infections were caused by MDR pathogens [12]. Hence, a risk-profile-based approach to select empiric antimicrobial therapy may have become less appropriate in critically ill patients with severe infections, and in nosocomial infections in particular. As an alternative, it has been suggested to guide empiric therapy on surveillance cultures [13, 14]. The past decade saw an increased interest in the potential of surveillance cultures to predict pathogens in severe nosocomial infections, mostly bacteraemia and VAP [15–18]. The correlation between surveillance cultures of the lower respiratory tract and diagnostic cultures at the time of clinical VAP has been extensively studied. However, to date, the literature remains inconclusive as study results differ substantially.
We performed a systematic review and a diagnostic test accuracy meta-analysis to analyse the sensitivity and specificity of surveillance cultures (prior to VAP onset) in predicting pathogens in VAP in adult patients.
Methods
A systematic review of the literature and a diagnostic test accuracy meta-analysis were performed [19]. The latter pools both the sensitivity and specificity of surveillance cultures. Hence, in a cohort of patients who developed VAP, for each patient, the microorganisms in the ‘surveillance’ culture(s) (prior to clinical suspicion of VAP) were compared with pathogens in the ‘diagnostic’ culture (on the day of VAP onset) [19]. The results are reported in accordance with the PRISMA guidelines (‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’).
Data sources and searches
The search string in PubMed and Web of Science databases consisted of two parts: (1) the infection of interest: ventilator-associated pneumonia (as MeSH or as part of the title including the following alternatives: ‘VAP’, ‘nosocomial pneumonia’, or ‘ventilated/ventilator/ventilation’); (2) combined with at least one of the following terms: ‘culture(s)’, ‘surveillance’, ‘colonised/colonisation/colonising’, ‘aspirate(s)’, ‘sample(s)’, or ‘screening’. Complementary searches were performed through analyses of reference lists, the Science Citation index, Cochrane library, CINAHL, and Google™, and searching for relevant publications of ‘expert’ authors (known or identified to have published in the field of surveillance cultures and/or VAP).
Study selection
The selection and assessment of eligibility were performed by three investigators (N.B., S.L., and S.B.) by mutual consideration based on the availability of all necessary data and accordance with in- and exclusion criteria. No language restrictions were applied, and the time period was not bounded.
We included studies providing original data on the accuracy of lower respiratory tract surveillance cultures to predict the bacterial pathogens in either clinically suspected or confirmed VAP in adult ICU patients. Relevant surveillance cultures were samples of the lower respiratory tract (all sampling techniques) obtained before clinical suspicion of VAP. Studies reporting only gram stains were excluded. Surveillance cultures sampled from other body sites were not considered (wound cultures, blood samples, oral, oropharynx, or nasal cultures).
Ventilator-associated pneumonia onset was based on clinical, microbiological, and/or radiological criteria. Studies reporting ≤5 patients with VAP were excluded, as were studies on fungal or viral pneumonia, and cohorts containing only tracheotomised, chronically ventilated patients. Case reports and review articles were not included.
Studies were only eligible if they contained all necessary data for the meta-analysis (see below). No language restrictions were applied, and the time period was not bounded.
Data extraction and quality assessment
The following data were collected (where available): basic study characteristics (author, year of publication, study period, setting, study design); population characteristics; in- and exclusion criteria; diagnostic criteria for VAP; microbiological workup of surveillance and diagnostic VAP cultures (type and frequency of sampling, culture techniques, thresholds for pathogen detection, and antimicrobial susceptibility).
Assessment of quality and generalisability was adapted from the QUADAS (‘Quality Assessment of Diagnostic Accuracy Studies’) guidelines for diagnostic test accuracy meta-analyses [19].
Due to the important variation in diagnostic techniques and definitions of VAP, the quality of VAP diagnosis was assessed according to the clarity of the description of clinical parameters (e.g. clinical pulmonary infection score), availability of radiological confirmation, and use of microbiological diagnostic techniques such as bronchoalveolar lavage (BAL) [20].
Data synthesis and analysis
Studies included in the diagnostic test accuracy meta-analyses were required to report the following variables: true positives (TP, pathogen(s) predicted by surveillance culture and retrieved in the diagnostic VAP culture), true negatives (TN, negative both in surveillance and VAP culture), false positives (FP, isolated from the surveillance culture but not from the diagnostic VAP culture), and false negatives (FN, isolated from the diagnostic VAP culture but not from the surveillance culture). If not directly reported, these data had to be retrievable through calculation. These predictive variables were used to calculate sensitivity, specificity, and positive and negative likelihood ratios, based on the total number of VAP episodes. Positive and negative predictive values are strongly dependent on prevalence (which varied between the included studies) and are thus less useful than sensitivity and specificity in evaluating the inherent test accuracy [21]. Therefore, a pooled estimate is not reported.
Subanalyses were conducted for VAP episodes caused by Staphylococcus aureus, Pseudomonas spp., and Acinetobacter spp., MDR microorganisms, frequency of sampling, and consideration of all versus only the most recent surveillance culture. As such, several sets of variables (TP, FP, FN, TN) could be subtracted from a single study. Therefore, in some analyses, the total number of variable sets may be higher than the number of articles included.
A variable set could be included in the mathematical model on the condition that the sum of its TP + FN ≥ 4. Candida spp., yeasts, coagulase-negative staphylococci, alpha-haemolytic streptococci, and diphtheroids are considered non-pathogenic and therefore were not taken into account [22–24].
Except for those variable sets assessing methicillin-sensitive S. aureus (MSSA), and S. aureus (irrespective of antimicrobial susceptibility), all variable sets considered potentially MDR microorganisms since the assumed potential of surveillance to predict MDR pathogens is of particular value in clinical practice. All studies were screened for definitions of MDR (if reported). A mathematical correction of the total number of VAP episodes was indispensable in ‘polymicrobial’ VAP episodes (counted as separate VAP episodes) in order to avoid underrepresentation of true negatives.
Statistical methods
A bivariate mixed-effect regression framework was used to perform the meta-analysis in STATA/MP4 (release 11; StataCorp LP, College Station, TX, USA; STATA, ‘mais’ and ‘midas’ modules). The area under the curve (AUC) of the hierarchical summary receiver operating characteristics (sROC) curve is a measure of the overall test performance. It summarises sensitivity and specificity in one value ranging between 0.0 and 1.0, with values <0.50 representing no predictive benefit of the surveillance cultures, values ranging 0.50–0.70 low accuracy, 0.70–0.90 moderate accuracy, and >0.90 high accuracy.
Uncertainty is quantified by 95 % confidence intervals (95 % CI). Test reproducibility and heterogeneity are assessed by means of forest plot analyses (not shown), and inconsistency is represented as I 2, with values >50 % representing substantial heterogeneity. The Fagan plot (or Bayes nomogram) visualises the clinical relevance of surveillance cultures using the likelihood ratios to calculate post-test probability based on Bayes’ theorem. In this statistical approach the pre-test probability represents the baseline risk of MDR involvement in VAP based on the prevalence of MDR in the studies.
A univariable meta-regression is used to evaluate the impact of potential confounders: MDR, sampling frequency, and consideration of either all or only the most recent surveillance culture.
Results
General description of included studies
The literature search was finalised 18 January 2012 and is shown in Fig. 1 (online supplement 1). Of all 525 studies, 35 full-text articles and 2 abstracts appeared suitable for inclusion [13, 14, 22–54]. Of these, 21 studies did not match our inclusion criteria (missing necessary data, only reported on total of mechanically ventilated patients, abstract also published as full paper) [13, 14, 24–26, 28, 31, 33, 41–54]. Only 14 studies, all in English and published after 1994, reported the predictive variables in sufficient detail to be included in the meta-analysis [22, 23, 27, 29, 30, 32–40]. The median study duration was 23 months (range 12–99 months) (not reported in two studies). In total, 791 VAP episodes (in over 688 patients) could be included (mean 57 episodes/study, range 11–151). Incidence of VAP was only reported in three studies: 10.4/1000 [39], 22.9/1000 [36], and 55/1000 [29] mechanical ventilation days; the latter was a study with burned patients with inhalation injury [29]. Prevalence could be extracted from nine studies and ranged between 7.3 and 44.6 % of all ventilated patients [22, 23, 30, 33, 34, 36–39].
Assessment of quality and generalisability is summarised in the table (online supplement 2). Nine studies described a mixed ICU cohort [23, 27, 30, 32, 34, 36–38, 40] and two a sample of medical ICU patients [35, 39]. The other studies involved surgical ICU patients [22], patients with head trauma or stroke [33], or mechanically ventilated burn patients with inhalation injury [29].
Except for one study [33], all studies defined a minimal duration of mechanical ventilation before VAP onset of ≥48 h [22, 23, 29, 30, 32, 35, 36, 39, 40], ≥72 h [27, 34, 38], or ≥96 h [37].
Sampling techniques and microbiological analysis (Table: online supplement 2)
In one study [34], surveillance cultures were obtained by protected specimen brushes and mini-BAL/non-directed bronchial lavage and in all 13 others by endotracheal aspirates. Three studies reported quantitative thresholds for positivity [33, 36, 39].
Seven studies reported multiple sampling techniques for diagnostic VAP cultures [22, 32–35, 37, 40]. Endotracheal aspirates were used in eight [22, 23, 29, 32, 33, 36, 37, 40], protected specimen brushes in five [30, 33–35, 37], and BAL in ten studies (performed in all patients in five studies [27, 30, 34, 38, 39]) [22, 32, 35, 37, 40]. Three studies only used semiquantitative microbiological techniques [27, 29, 32].
In only four studies [27, 29, 32, 39], a definition of MDR was reported and used as such in the meta-analysis. In the remaining ten studies, microorganisms were considered MDR in case of methicillin-resistant S. aureus (MRSA), Pseudomonas spp., or Acinetobacter spp.
Meta-analysis
Depending on the respective research question, a total of 42 sets of predictive variables (TP, FP, TN, and FN) could be identified from the 14 studies, e.g. prediction of MRSA, prediction of ‘MDR’. Two studies reported both the predictive value for ‘all’ and the ‘most recent’ surveillance cultures [34, 37]. From these studies, the sets containing ‘all’ surveillance cultures were used in the calculations (7 sets in total) [34, 37]. The seven corresponding sets of variables based on the ‘most recent’ surveillance cultures were only used in the univariate analysis to assess their impact on the accuracy of prediction (thus 49 sets were used for univariable analysis) [34, 37].
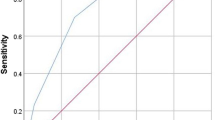
An sROC curve visualises the inter-study variation in sensitivity and specificity among the 42 variable sets and demonstrates high accuracy (AUC = 0.92; 95 % CI: 0.89–0.94) (Fig. 1a). A second sROC curve (Fig. 1b) summarises the ability of surveillance cultures to predict MDR pathogens using only one variable set per study (n = 14). This sROC curve shows less variability (data points closer to the pooled value) but similar discriminative power (AUC = 0.90; 95 % CI: 0.87–0.92). A forest plot (Fig. 2) represents the sensitivity and specificity for each of these 14 variable sets. All sub-analyses are presented in Table 1. For five out of six subanalyses the accuracy is high (AUC > 0.90). Only for the prediction of MRSA is the accuracy judged to be moderate (AUC = 0.83). However, pooled specificity for this subanalysis is particularly high (0.98). The highest accuracy is seen for the four studies reporting an overall prediction of MDR. This subanalysis also shows the least statistical heterogeneity (I 2 = 63).
Between-study heterogeneity as visualised by a summary receiver-operating characteristics (sROC) curve with confidence and prediction regions around the mean operating sensitivity and specificity point for surveillance cultures to predict bacterial pathogens in VAP. a All different variations (n = 42); b predicting multidrug resistance—one per study (n = 14*). AUC area under the curve, SENS sensitivity, SPEC specificity, sROC summary receiver-operating characteristics.*Reference numbers of the 14 included studies: [21, 22, 26, 28, 29, 31–39]
A Fagan plot is constructed to illustrate the pre-test and post-test probability of surveillance cultures to predict MDR involvement based on all 14 studies (Fig. 3a). Not taking into account surveillance cultures, a VAP episode has a (‘pre-test’) probability of 25 % to be caused by MDR pathogen(s). With a surveillance culture positive for an MDR microorganism, there is a 75 % “post-test” probability of a subsequent VAP episode to be caused by the same MDR pathogen. With a negative surveillance culture, the post-test probability of VAP with an MDR pathogen drops to 8 %. When the Fagan plot is constructed for the four studies clearly defining MDR [27, 29, 32, 39], positive and negative post-test probabilities are 82 and 5 % respectively (Fig. 3b).
Fagan plot: pre-test and post-test likelihood for ventilator-associated pneumonia to be caused by multidrug-resistant pathogens. The pre-test (prior) probability is the probability of a VAP episode to be caused by MDR pathogens (without taking into account the test/surveillance cultures). The post-test probability takes into account the results of the surveillance cultures, which do or do not contain MDR pathogens (predicting respectively positive and negative likelihood). a All 14 studies* (MDR as defined in methods); b 4 studies** that clearly reported the predictive value of all MDR microorganisms in the surveillance cultures. *Reference numbers of the 14 included studies: [21, 22, 26, 28, 29, 31–39]. **Reference numbers of the 4 included studies: [26, 28, 31, 38]
Factors influencing the predictive accuracy of surveillance cultures (univariable analyses)
Three factors are reported in sufficient detail to assess their influence on the accuracy of prediction. First, the data are insufficiently detailed and too heterogeneous to analyse the impact of antimicrobial susceptibility patterns in Pseudomonas and Acinetobacter spp. However, the data of six studies could be used to compare predictions for MSSA versus MRSA. Sensitivity is slightly higher for MRSA than for MSSA [0.75 (0.95 % CI: 0.58–0.91) vs. 0.70 (0.95 % CI: 0.48–0.92)], although specificity is similar 0.97 (0.95 % CI: 0.92–1.00) versus 0.97 (0.95 % CI: 0.91–1.00).
Sensitivity for MRSA (0.75, 95 % CI: 0.58–0.91) is slightly higher than for MSSA (0.70, 95 % CI: 0.48–0.92), although specificity is similar: 0.97 (0.95 % CI: 0.92–1.00) versus 0.97 (95 % CI: 0.91–1.00) for MRSA and MSSA respectively.
Second, the univariable analysis shows improved sensitivity (0.79; 95 % CI: 0.74–0.85 vs. 0.65; 95 % CI: 0.57–0.72) and specificity (0.96; 95 % CI: 0.94–0.98 vs. 0.90; 95 % CI: 0.86–0.95) when only the most recent surveillance cultures are taken into account instead of all cultures (Fig. 4).
Finally, a sampling frequency of at least twice a week results in better sensitivity (0.78; 95 % CI: 0.73–0.83 vs. 0.61; 95 % CI: 0.52–0.69) and specificity (0.96; 95 % CI: 0.94–0.98 vs. 0.87; 95 % CI: 0.82–0.93) compared with less frequent sampling. A sampling frequency of thrice weekly does not further strengthen the predictive accuracy of surveillance cultures (Fig. 4).
Discussion
Although statistically challenging, this diagnostic test accuracy meta-analysis provides coherent evidence for the accuracy of surveillance cultures of the respiratory tract to predict bacterial pathogens in VAP, in particular the absence of multidrug-resistant pathogens. Despite the relatively low number of patients and studies included in some of the sub-analyses, pooled sensitivities (between 0.72 and 0.84) and specificities (between 0.90 and 0.98) correspond with moderate to high accuracy (AUC between 0.83 and 0.95). Although the incidence of VAP varies among the studies, this does not impact the results, which are only based on patients with VAP. However, a great variety of VAP diagnoses, MDR assessments, and treatment policies was present in the different studies.
The most important clinical implication is the high specificity and negative likelihood of surveillance cultures. If a recent surveillance culture does not contain MDR microorganisms, the newly symptomatic VAP is unlikely to be caused by MDR (likelihood <10 %) (Fig. 3). The high specificity of surveillance cultures increases its antibiotic-saving potential as it delineates patients in which extensive broad-spectrum antibiotic therapy may be unnecessary. Surveillance-assisted empiric therapy has been associated with an effective reduction in antibiotic consumption by comparing it with hypothetical, strictly empirical antimicrobial schemes [32, 39, 55]. Thus, if surveillance cultures are used to guide antibiotic consumption, an emphasis on multidrug-resistant microorganisms appears to be recommendable, but clinical evaluation of the patient for signs of infection remains crucial.
The presence of MDR microorganisms in surveillance cultures of the lower respiratory tract predicts the pathogen(s) with a 75–82 % confidence rate, which justifies antimicrobial coverage. Particular emphasis must be given to the sampling frequency. A once weekly sampling frequency might be of little value to steer empiric antimicrobial therapy in VAP. Our analyses suggest that twice-weekly sampling is to be preferred above less frequent sampling (Fig. 4). No additional benefit could be demonstrated for thrice weekly sampling. This could be due to the low number of studies using thrice-weekly sampling but also because in twice-weekly sampling specificity already reaches 96 %, leaving little room for improvement.
It also appears that it is more accurate to only consider the most recent surveillance culture, as both sensitivity and specificity drop when all surveillance cultures are taken into account. From the dynamics of colonisation preceding infection, a higher number of true positives and a lesser number of false negatives are to be expected when considering only the most recent surveillance cultures, as the relative weight of relevance of a positive surveillance culture is likely to depend on the timeframe between sampling and VAP onset, as well as the sampling frequency. This could, however, not be determined in this study. Further prospective evaluation is thus necessary to determine the ideal sampling frequency and maximal time interval of relevance (especially considering the negative predictive value) between the last surveillance culture and VAP onset, which are both clinically important in view of the dynamic nature of the colonisation of the respiratory tree.
No other published systematic review has addressed the same research question with a similar methodological approach. Several of the included studies [27, 29, 32, 37–40] advocate the benefit of surveillance cultures, whereas others contradict this conclusion [24, 26, 28, 30, 35, 51]. Heterogeneity between studies may be explained by contrasting results. Clinical heterogeneity originates from differences in the patient case mix reflecting a distinct risk profile for VAP and/or MDR involvement (trauma, elective surgery, medical disease, antibiotic exposure, length of hospitalisation, etc.). Also differences in diagnostic techniques (invasive vs. non-invasive diagnosis of VAP) as well as microbiological techniques (quantitative vs. semi-quantitative techniques) might have influenced the findings in some studies. Heterogeneity is also due to differences in inclusion criteria and study designs (e.g., sampling frequency, which appeared to be of major importance). In this meta-analysis we attempted to limit heterogeneity by implementing strict inclusion criteria (e.g., only adult ICU populations) and by focusing on the clinical implications of surveillance cultures of the respiratory tract in VAP. Due to our strict inclusion criteria, only 14 out of 34 articles addressing surveillance cultures could be included in the meta-analysis, which should have contributed to reducing clinical heterogeneity and inter-study variability, and hence increased internal validity. In spite of this strict selection process, a substantial statistical heterogeneity remains (I 2 = 0–92 %), indicating an important remaining inter-study variation.
The apparent strength of our results in favour of surveillance cultures may be attributed to the focus on MDR pathogens. Evaluation of non-pathogenic microorganisms in surveillance or VAP cultures will also blur the results, since these generally represent colonisation. Broadening the study with surveillance cultures of all mechanically ventilated patients [14, 24–26, 41, 42, 51] (not only those who developed VAP) would also result in higher false-positive rates, as the correlation with diagnostic cultures in subsequent VAP is weakened by the inclusion of irrelevant information in the denominator. Therefore, we restricted our research question to patients with VAP. However, in these cohorts of VAP patients, a high proportion of MDR was found (Table 1), which might hamper generalisability to ICUs with lower rates of MDR.
We only focussed on lower respiratory tract surveillance cultures. Taking into account surveillance cultures sampled from other body sites tends to increase sensitivity while decreasing specificity [35, 55]. In a study on nosocomial pneumonia (predominantly VAP), the proportion of MDR pathogens predicted by either tracheal or all surveillance cultures (additional urine, rectal and oral swabs) was respectively 70 and 88 % [55]. By considering all body sites, however, the proportion of false positives tripled from 15 to 46 % [55]. This weakens the high specificity required in a surveillance culture approach.
Despite the fact that meta-analyses should be considered as the best available evidence, they rely on the quality of the available data, which is in this case subject to enormous variation. Substantial controversy remains considering the ideal sampling and microbiological techniques and thresholds [56]. Therefore, ascertainment bias is a possible limitation of our study (e.g. on the degree of certainty of a true diagnosis of VAP), since some studies might have examined a more ‘severe’ VAP-patient cohort than others. In addition, an incorporation bias could have occurred, since several studies used the same sampling technique—e.g. endotracheal aspirates—for both surveillance and diagnostic cultures. Perhaps the impact of this bias is limited because these endotracheal aspirates are sampled at different time points, and clinical samples are generally analysed using different microbiological thresholds [57]. Nevertheless, a subgroup analysis exclusively including studies that used endotracheal aspirates as surveillance cultures and bronchoscopy-based diagnosis of VAP would have strengthened the study. Yet, for this purpose, only five studies adding up for 232 VAP episodes could be considered, and this proved to be insufficient for execution of a diagnostic test accuracy meta-analysis. Because the impact of using different diagnostic approaches could not be evaluated, the results of this meta-analysis must be interpreted with caution as a diagnosis of VAP based on non-invasive techniques includes a substantial risk of false-positive results [51].
Due to the limited number of studies (often leading to small subgroups), and important inter-study variation, our results should certainly be interpreted with caution. Unfortunately, the available data did not allow further exploration, since more detailed reporting would merely reflect the results of individual studies instead of the combined assessment of several sources of ‘evidence’, which is the aim of a meta-analysis. It is for example expected that several factors such as previous antibiotic exposure, antimicrobial policies, and duration of mechanical ventilation influence the predictive value of surveillance cultures. However, no recommendations could be made based on this meta-analysis.
Another limitation of the study is that only four studies provided detailed definitions of MDR. In the analysis with all studies included, only MRSA, Pseudomonas, and Acinetobacter species were considered MDR. With the exception of the latter two non-fermenting bacteria, no other MDR gram negatives were considered because most studies did not discriminate for instance between extended-spectrum beta-lactamase (ESBL)- and non-ESBL-producing gram-negative bacteria in their report.
Although it could be expected that surveillance cultures are particularly useful in ICUs with a high MDR incidence, cost-effectiveness remains to be evaluated, since this is an important argument against routine surveillance cultures, which are related with a high workload, especially for the laboratory. Thus, the balance between benefits and costs remains to be evaluated.
Besides surveillance cultures, real-time PCR might also provide information to steer empiric antimicrobial therapy. This technology enables bacterial identification in less than 1 h. A drawback of this technique is that it does not allow discriminating colonisation from infection, and since VAP is frequently caused by microorganisms colonising the upper respiratory tract, the added value remains limited [58]. However, in the past decade, progress in the development of molecular diagnostics has been spectacular, and the future might bring technologies to identify causative pathogens in VAP with susceptibility patterns within a clinically more acceptable time frame [58].
In conclusion, this study underscores the value of surveillance cultures of the lower respiratory tract in predicting bacterial pathogens in VAP in adult ICU patients, in particular for the absence of MDR pathogens. However, heterogeneity and bias remain important limitations of this meta-analysis.
References
Safdar N, Dezfulian C, Collard HR, Saint S (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 33:2184–2193
Blot S, Depuydt P, Vogelaers D (2008) Maximizing rates of empiric appropriate antibiotic therapy with minimized use of broad-spectrum agents: are surveillance cultures the key? Intensive Care Med 34:2130–2133
Timsit JF, Zahar JR, Chevret S (2011) Attributable mortality of ventilator-associated pneumonia. Curr Opin Crit Care 17:464–471
Kollef M (2003) Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time. Drugs 63:2157–2168
Blot S, Vandewoude K (2004) Early detection of systemic infections. Acta Clin Belg 59:20–23
Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly EC (1997) Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 111:676–685
Kollef MH, Ward S (1998) The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest 113:412–420
Kollef MH (1999) Antimicrobial therapy of ventilator-associated pneumonia: how to select an appropriate drug regimen. Chest 115:8–11
Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, Gibert C (1998) Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 157:531–539
Masterton RG (2009) The new treatment paradigm and the role of carbapenems. Int J Antimicrob Agents 33:105–110
Blot S (2008) Limiting the attributable mortality of nosocomial infection and multidrug resistance in intensive care units. Clin Microbiol Infect 14:5–13
Vogelaers D, De Bels D, Foret F, Cran S, Gilbert E, Schoonheydt K, Blot S (2010) Patterns of antimicrobial therapy in severe nosocomial infections: empiric choices, proportion of appropriate therapy, and adaptation rates–a multicentre, observational survey in critically ill patients. Int J Antimicrob Agents 35:375–381
Bryant LR, Trinkle JK, Mobin-Uddin K, Griffen WO Jr (1972) Interpretation of tracheal cultures in patients with intubation and mechanical ventilation. Am Surg 38:537–541
Polk HC Jr (1975) Quantitative tracheal cultures in surgical patients requiring mechanical ventilatory assistance. Surgery 78:485–491
Baba H, Nimmo GR, Allworth AM, Boots RJ, Hayashi Y, Lipman J, Paterson DL (2011) The role of surveillance cultures in the prediction of susceptibility patterns of Gram-negative bacilli in the intensive care unit. Eur J Clin Microbiol Infect Dis 30:739–744
Blot S, Depuydt P, Vogelaers D, Decruyenaere J, De Waele J, Hoste E, Peleman R, Claeys G, Verschraegen G, Colardyn F, Vandewoude K (2005) Colonization status and appropriate antibiotic therapy for nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in an intensive care unit. Infect Control Hosp Epidemiol 26:575–579
Reddy P, Malczynski M, Obias A, Reiner S, Jin N, Huang J, Noskin GA, Zembower T (2007) Screening for extended-spectrum beta-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bacteremia. Clin Infect Dis 45:846–852
Depuydt P, Benoit D, Vogelaers D, Claeys G, Verschraegen G, Vandewoude K, Decruyenaere J, Blot S (2006) Outcome in bacteremia associated with nosocomial pneumonia and the impact of pathogen prediction by tracheal surveillance cultures. Intensive Care Med 32:1773–1781
Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM (2008) Systematic reviews of diagnostic test accuracy. Ann Intern Med 149:889–897
Koulenti D, Lisboa T, Brun-Buisson C, Krueger W, Macor A, Sole-Violan J, Diaz E, Topeli A, DeWaele J, Carneiro A, Martin-Loeches I, Armaganidis A, Rello J (2009) Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med 37:2360–2368
Jones CM, Ashrafian H, Skapinakis P, Arora S, Darzi A, Dimopoulos K, Athanasiou T (2010) Diagnostic accuracy meta-analysis: a review of the basic principles of interpretation and application. Int J Cardiol 140:138–144
Nair S, Sen N, Peter JV, Raj JP, Brahmadathan KN (2008) Role of quantitative endotracheal aspirate and cultures as a surveillance and diagnostic tool for ventilator associated pneumonia: a pilot study. Indian J Med Sci 62:304–313
de Latorre FJ, Pont T, Ferrer A, Rossello J, Palomar M, Planas M (1995) Pattern of tracheal colonization during mechanical ventilation. Am J Respir Crit Care Med 152:1028–1033
Gursel G, Aydogdu M, Nadir Ozis T, Tasyurek S (2010) Comparison of the value of initial and serial endotracheal aspirate surveillance cultures in predicting the causative pathogen of ventilator-associated pneumonia. Scand J Infect Dis 42:341–346
Albert S, Kirchner J, Thomas H, Behne M, Schur J, Brade V (1997) Role of quantitative cultures and microscopic examinations of endotracheal aspirates in the diagnosis of pulmonary infections in ventilated patients. J Hosp Infect 37:25–37
Aydogdu M, Gursel G, Hizel K, Ozis TN (2010) Comparison of the serial surveillance with quantitative and non-quantitative tracheal aspirate in predicting ventilator-associated pneumonia etiology in patients receiving antibiotic therapy. Minerva Anestesiol 76:600–608
Bagnulo H, Godino M, Galiana A, Bertulo M, Pedreira W (2007) Are routine endotracheal aspirates predictive of the etiology of ventilator-associated pneumonia? Crit Care 11(Suppl 2):p 87
Bouza E, Perez A, Munoz P, Jesus Perez M, Rincon C, Sanchez C, Martin-Rabadan P, Riesgo M (2003) Ventilator-associated pneumonia after heart surgery: a prospective analysis and the value of surveillance. Crit Care Med 31:1964–1970
Brusselaers N, Logie D, Vogelaers D, Monstrey S, Blot S (2012) Burns, inhalation injury and ventilator-associated pneumonia: value of routine surveillance cultures. Burns 38:364–370
Cendrero Cardenosa JA, Sole-Violan J, Bordes Benitez A, Noguera Catalan J, Arroyo Fernandez J, Saavedra Santana P, Rodriguez de Castro F (1999) Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest 116:462–470
Delclaux C, Roupie E, Blot F, Brochard L, Lemaire F, Brun-Buisson C (1997) Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome: incidence and diagnosis. Am J Respir Crit Care Med 156:1092–1098
Depuydt P, Benoit D, Vogelaers D, Decruyenaere J, Vandijck D, Claeys G, Verschraegen G, Blot S (2008) Systematic surveillance cultures as a tool to predict involvement of multidrug antibiotic resistant bacteria in ventilator-associated pneumonia. Intensive Care Med 34:675–682
Ewig S, Torres A, El-Ebiary M, Fabregas N, Hernandez C, Gonzalez J, Nicolas JM, Soto L (1999) Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med 159:188–198
Flanagan PG, Findlay GP, Magee JT, Ionescu A, Barnes RA, Smithies M (2000) The diagnosis of ventilator-associated pneumonia using non-bronchoscopic, non-directed lung lavages. Intensive Care Med 26:20–30
Hayon J, Figliolini C, Combes A, Trouillet JL, Kassis N, Dombret MC, Gibert C, Chastre J (2002) Role of serial routine microbiologic culture results in the initial management of ventilator-associated pneumonia. Am J Respir Crit Care Med 165:41–46
Joseph NM, Sistla S, Dutta TK, Badhe AS, Parija SC (2010) Ventilator-associated pneumonia: role of colonizers and value of routine endotracheal aspirate cultures. Int J Infect Dis 14:e723–e729
Lampati L, Maggioni E, Langer M, Malacarne P, Mozzo R, Pesenti A, Fumagalli R (2009) Can routine surveillance samples from tracheal aspirate predict bacterial flora in cases of ventilator-associated pneumonia? Minerva Anestesiol 75:555–562
Malacarne P, Corini M, Maremmani P, Viaggi B, Verdigi S (2007) Diagnostic characteristics of routine surveillance cultures of endotracheal aspirate samples in cases of late-onset ventilator-associated pneumonia due to Acinetobacter baumannii. Infect Control Hosp Epidemiol 28:867–869
Michel F, Franceschini B, Berger P, Arnal JM, Gainnier M, Sainty JM, Papazian L (2005) Early antibiotic treatment for BAL-confirmed ventilator-associated pneumonia: a role for routine endotracheal aspirate cultures. Chest 127:589–597
Papadomichelakis E, Kontopidou F, Antoniadou A, Poulakou G, Koratzanis E, Kopterides P, Mavrou I, Armaganidis A, Giamarellou H (2008) Screening for resistant gram-negative microorganisms to guide empiric therapy of subsequent infection. Intensive Care Med 34:2169–2175
Sirvent JM, Torres A, Vidaur L, Armengol J, de Batlle J, Bonet A (2000) Tracheal colonisation within 24 h of intubation in patients with head trauma: risk factor for developing early-onset ventilator-associated pneumonia. Intensive Care Med 26:1369–1372
Valles J, Mariscal D, Cortes P, Coll P, Villagra A, Diaz E, Artigas A, Rello J (2004) Patterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumonia. Intensive Care Med 30:1768–1775
Yang K, Zhuo H, Guglielmo BJ, Wiener-Kronish J (2009) Multidrug-resistant Pseudomonas aeruginosa ventilator-associated pneumonia: the role of endotracheal aspirate surveillance cultures. Ann Pharmacother 43:28–35
A’Court CH, Garrard CS, Crook D, Bowler I, Conlon C, Peto T, Anderson E (1993) Microbiological lung surveillance in mechanically ventilated patients, using non-directed bronchial lavage and quantitative culture. Q J Med 86:635–648
Bergmans DC, Bonten MJ, Stobberingh EE, van Tiel FH, van der Geest S, de Leeuw PW, Gaillard CA (1998) Colonization with Pseudomonas aeruginosa in patients developing ventilator-associated pneumonia. Infect Control Hosp Epidemiol 19:853–855
Boots RJ, Phillips GE, George N, Faoagali JL (2008) Surveillance culture utility and safety using low-volume blind bronchoalveolar lavage in the diagnosis of ventilator-associated pneumonia. Respirology 13:87–96
Fussle R, Biscoping J, Zeiler D, Michaelis G, Sziegoleit A (1991) Microbiologic monitoring of ventilated intensive-care patients: a concept for diagnosis and therapy of pulmonary infections. Anaesthesist 40:491–496
George DL, Falk PS, Wunderink RG, Leeper KV Jr, Meduri GU, Steere EL, Corbett CE, Mayhall CG (1998) Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med 158:1839–1847
Jung B, Sebbane M, Chanques G, Courouble P, Verzilli D, Perrigault PF, Jean-Pierre H, Eledjam JJ, Jaber S (2009) Previous endotracheal aspirate allows guiding the initial treatment of ventilator-associated pneumonia. Intensive Care Med 35:101–107
Konrad F, Heeg K, Wiedeck H, Kilian J (1990) Routine throat swabs in artificially ventilated patients: meaningful bacteriologic monitoring or a needless procedure? Anaesthesist 39:323–329
Sanders KM, Adhikari NK, Friedrich JO, Day A, Jiang X, Heyland D (2008) Previous cultures are not clinically useful for guiding empiric antibiotics in suspected ventilator-associated pneumonia: secondary analysis from a randomized trial. J Crit Care 23:58–63
Wear RE, Morrow LE (2007) Quantitative surveillance tracheal aspirate dynamics as a predictor of ventilator-associated pneumonia. Crit Care Med 35:796
Zhuo H, Yang K, Lynch SV, Dotson RH, Glidden DV, Singh G, Webb WR, Elicker BM, Garcia O, Brown R, Sawa Y, Misset B, Wiener-Kronish JP (2008) Increased mortality of ventilated patients with endotracheal Pseudomonas aeruginosa without clinical signs of infection. Crit Care Med 36:2495–2503
Logie D, Brusselaers N, Monstrey S, Vogelaers D, Blot S (2010) Incidence of ventilator-associated pneumonia in burn patients with inhalation injury and the value of routine endotracheal aspirate surveillance cultures to predict involvement of multidrug resistant microbial etiology. Acta Clin Belg 65:461
Depuydt PO, Blot SI, Benoit DD, Claeys GW, Verschraegen GL, Vandewoude KH, Vogelaers DP, Decruyenaere JM, Colardyn FA (2006) Antimicrobial resistance in nosocomial bloodstream infection associated with pneumonia and the value of systematic surveillance cultures in an adult intensive care unit. Crit Care Med 34:653–659
Berton DC, Kalil AC, Teixeira PJ (2012) Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev 1: CD006482
Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM (2006) Evidence of bias and variation in diagnostic accuracy studies. CMAJ 174:469–476
Munoz P, Cercenado E, Giannella M, Bouza E (2009) Rapid detection of micro-organism resistance in patients with ventilator-associated pneumonia. Clin Pulm Med 16:302–308
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brusselaers, N., Labeau, S., Vogelaers, D. et al. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med 39, 365–375 (2013). https://doi.org/10.1007/s00134-012-2759-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2759-x